Introduction
Follicular lymphoma (FL) is the second most common non-Hodgkin lymphoma. Leukemic-phase FL (LP-FL) is a rare entity comprising 1-3% of FL patients (pts). Controversy exists about prognosis of LP-FL with some studies suggesting worse outcome. No clear cut-point of circulating cells has been used to define LP-FL; however, a level > 5x103^3 cells/uL is defined in GELF criteria for high burden disease. No unified treatment recommendation is present for these pts with some opting to treat as indolent FL and some choosing a more aggressive approach. Furthermore, limited case series (n=7 to 37) on LP-FL are available, hence we aimed to better understand prognosis and treatment strategies associated with better outcome in LP-FL.
Methods
Pts with a pathology proven diagnosis of FL in lymph node and/or bone marrow and a positive flow cytometry in peripheral blood for clonal B cells (CBs), at diagnosis or at relapse, were included in the study. Data was collected from 5 academic institution in the US. We report time-to-event outcomes including median progression-free survival (mPFS) and overall survival (mOS) estimated by Kaplan-Meier method (only pts who received therapy included) . Uni and multivariable analyses were performed using Cox regression. CBs were divided into 4 groups: (<1, 1-4.99, 5-9.99, >10) 10^3/uL. Pts treatment was grouped in 4 categories: anti-CD20 monoclonal antibody (CD20mAb), CD20mAb+Bendamustine, CD20mAb+ CHOP, and other. Pts with CD10 negative clones on flow cytometry were excluded as they might represent different lymphoma type.
Results
Sixty-one pts (period 1998 to 2022) were identified in participating institutions with LP-FL, 10 of which had CD10- flow cytometry and excluded from study. Of 51 remaining pts, median age was 56 years (y). Nine (17.6%) pts were Hispanic and 44 (86.2%) pts were white. Most pts (37%) presented asymptomatic lymphadenopathy, 22% with B symptoms, 14% with incidental elevated WBC, 12% with organ compromise and 12% due to other reasons. Overall, B symptoms were present in 20 (39%) pts. Hepato/splenomegaly was present in 22 (43%) pts, high tumor burden (GELF criteria) in 12 (23%) pts, and most pts (n=42; 82%) presented ECOG PS 0-1. We less frequently observed anemia (Hg <12g/dL, n= 10; 20%) and elevated LDH (n=17; 33%). High risk FLIPI score (≥3) was present in 28 (55%) pts. Median count of CBs of 1.62 x 10^3/uL. CBs were <5 10^3/uL in 35 (69%) pts, CBs=5-10 10^3/uL in 6 (12%) pts and CBs >10 10^3/uL in 10 (20%) pts. Median absolute lymphocyte count was 3.0 x10^3/uL. Cytogenetic data was available in tissue biopsies in 13 (25%) pts and all carried t(14;18). Treatment was as follows: 24 (47%) pts with bendamustine + CD20mAb , 15 (29%) pts with CD20mAb + CHOP, 7 (14%) pts were treated with CD20mAb, 3 (6%) pts underwent active surveillance, and 2 (4%) with other treatments. Overall response rate (ORR) was 87%. Of those 31 (65%) pts had complete response (CR) and 11 (23%) pts had partial response (PR).
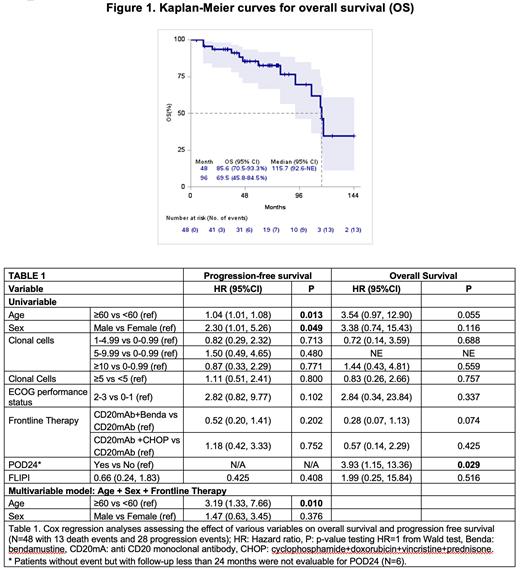
With a median follow up of 5.5 y the mPFS was 3.6 y and mOS was 9.6 y (Figure 1). Relapse/ progression of disease occurred in 26 (54%) pts and CBc was detect in 12/13 pts in which flow cytometry was done. Transformation on relapse occurred in 7 pts. In pts experiencing disease relapse/progression, CAR T-cell was given to 6 pts with an ORR of 83.3% (CR: in 4 and PR in 1 pts); one pt died due to CAR-T grade 5 neurotoxicity. Death occurred in 13 pts with 6 (46% of all deaths) being lymphoma-related.
Age≥60 and male sex were associated with worse PFS in univariable analysis (Table 1), with age only remaining significant in a multivariable analysis that also included sex and frontline therapy (HR 3.19, p=0.01 ). Notably, FLIPI score, CBs count category, and type of frontline therapy did not predict PFS. Progression of disease within 24 months (POD24) predicted OS in univariable analysis (HR= 3.93; p=0.029). Multivariable analysis for OS was not performed due to small number of deaths.
Conclusion
To our knowledge, this is the largest study of LP-FL. Median OS was around 10 years, a comparable outcome to historical nodal FL survival. Treatment type also did not predict PFS or OS. Circulating monoclonal cell levels did not impact survival . This is notable as the current GELF criteria includes circulating malignant cells ≥5 x10^3/uL as an indication for treatment while our date shows that no set cut-point may be needed. In addition, CAR T-cell appears to be an effective therapy with similar results as in nodal FL.
Disclosures
Trabolsi:Johnson & Johnson: Current equity holder in publicly-traded company. Epperla:Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Speakers Bureau. Ollila:ADC Therapeutics: Honoraria; Ono Pharmaceuticals: Honoraria, Research Funding. Karmali:Takeda: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Research Funding; Kite/Gilead: Consultancy, Honoraria, Research Funding; Miltenyi: Consultancy, Honoraria, Research Funding; Calithera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech/Roche: Consultancy, Honoraria; Lilly: Consultancy, Honoraria; Morphosys: Consultancy, Speakers Bureau; Janssen: Consultancy. Lossos:Adaptive: Honoraria; LRF: Membership on an entity's Board of Directors or advisory committees; NCI: Research Funding; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy. Alderuccio:Genmab: Research Funding; Abbvie: Consultancy; Genentech: Consultancy; ADC Therapeutics: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal